
The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. The term is most common in biochemistry, where it is a synonym of saccharide (from Ancient Greek σάκχαρον ( sákkharon) 'sugar' ), a group that includes sugars, starch, and cellulose. However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. It consists of a molecule of D-galactose and a molecule of D-glucose bonded by beta-1-4 glycosidic linkage.Ī carbohydrate ( / ˌ k ɑːr b oʊ ˈ h aɪ d r eɪ t/) is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula C m(H 2O) n (where m may or may not be different from n), which does not mean the H has covalent bonds with O (for example with CH 2O, H has a covalent bond with C but not with O). Maltose, Elmhurst College Virtual Chembook.Lactose is a disaccharide found in animal milk.Media related to Maltose at Wikimedia Commons.CRC Handbook of Food Additives, Second Edition. Grosch, Werner Schieberle, Peter (15 January 2009). ^ "150 Years Alfred Wöhlk :: Education :: ChemistryViews"."XXI.?On the transformation-products of starch". Chelsea, Michigan: Yale University Press. Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology. CRC Handbook of Chemistry and Physics (62nd ed.). A 10% solution of maltose is 35% as sweet as sucrose. It has a sweet taste, but is only about 30–60% as sweet as sugar, depending on the concentration. Maltose can easily be detected by the Woehlk test or Fearon's test on methylamine. Maltose in aqueous solution exhibits mutarotation, because the α and β isomers that are formed by the different conformations of the anomeric carbon have different specific rotations, and in aqueous solutions, these two forms are in equilibrium. Maltose can be broken down to glucose by the maltase enzyme, which catalyses the hydrolysis of the glycosidic bond.
#Anomeric carbon definition free#
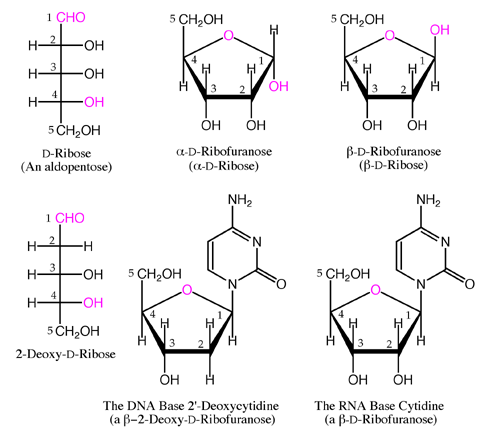
Like glucose, maltose is a reducing sugar, because the ring of one of the two glucose units can open to present a free aldehyde group the other one cannot because of the nature of the glycosidic bond. This is similar to maltose but instead of a bond in the α(1→4) position, it is in the α(1→6) position, the same bond that is found at the branch points of glycogen and amylopectin. The anomeric carbon (C 1) of the second glucose molecule, which is not involved in a glycosidic bond, could be either an α- or β-anomer depending on the bond direction of the attached hydroxyl group relative to the CHĢOH substituent of the same ring, resulting in either α-maltose or β-maltose. If the glycosidic bond to the anomeric carbon (C 1) were in the same plane as the CHĢOH substituent, it would be classified as a β(1→4) bond, and the resulting molecule would be cellobiose. The link is characterized as α because the glycosidic bond to the anomeric carbon (C 1) is in the opposite plane from the CHĢOH substituent in the same ring (C 6 of the first glucose). The two glucose units are in the pyranose form and are joined by an O-glycosidic bond, with the first carbon (C 1) of the first glucose linked to the fourth carbon (C 4) of the second glucose, indicated as (1→4). Glucose is a hexose: a monosaccharide containing six carbon atoms. Maltose, with two sugar units, is a disaccharide, which falls under oligosaccharides. Structure and nomenclature Ĭarbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the number of sugar subunits. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. An example of this reaction is found in germinating seeds, which is why it was named after malt. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose ( / ˈ m ɔː l t oʊ s/ or / ˈ m ɔː l t oʊ z/ ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. Amylase reaction consisting of hydrolyzing amylose, producing maltose


 0 kommentar(er)
0 kommentar(er)
